About PACE
In theory, biomolecular models containing every single atomic detail are the most accurate computational way to probe protein conformational dynamics. In practice, however, due to the expensive computational costs required for simulations with such details, all-atom models are oftentimes applicable only for simulating structural changes on a timescale far shorter than needed for observing biologically relevant events.
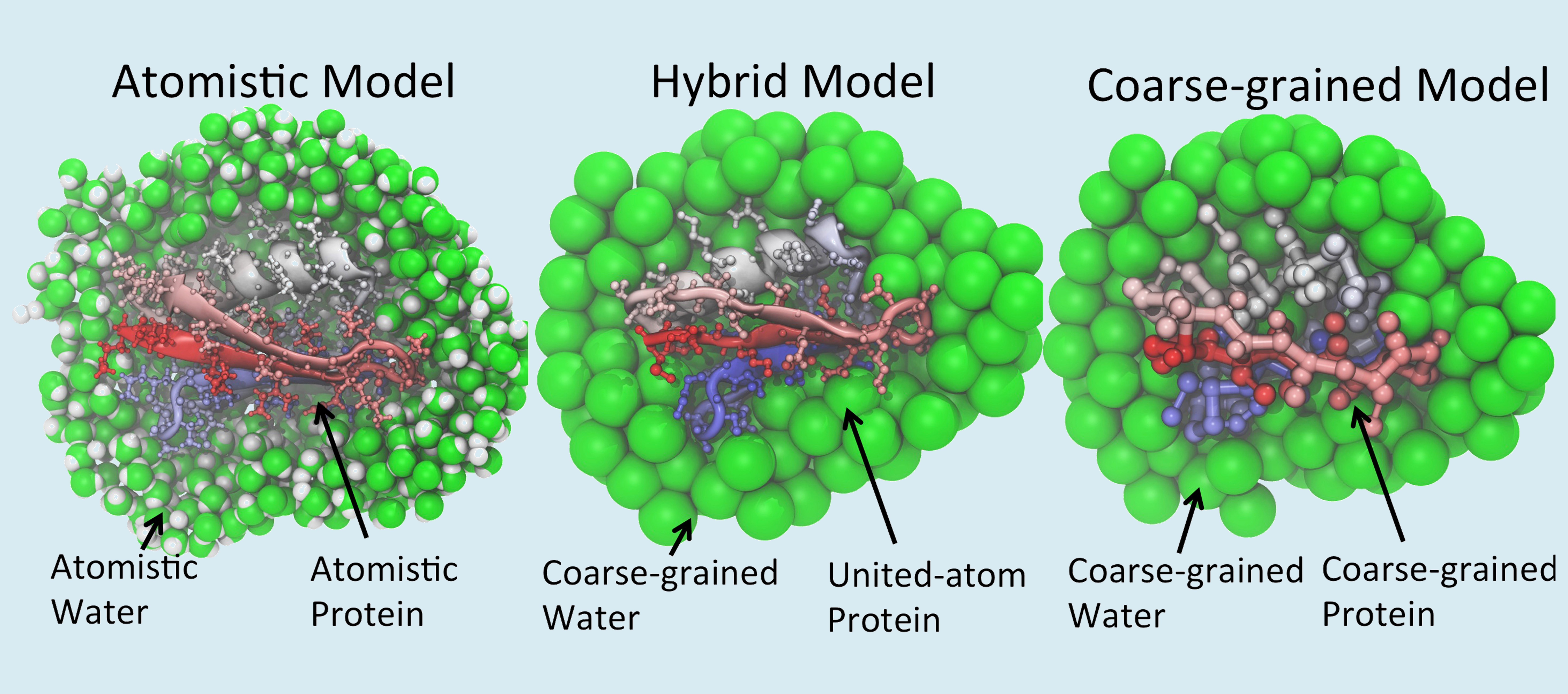
An appealing solution to this problem is to develop a model combining atomistic descriptions of proteins with CG environments. The model would permit the modeling of protein conformational dynamics with adequate detail but in a highly efficient manner. Our group have conceived and developed one such model, namely PACE (Protein with Atomic detail in Coarse-grained Environment), that enhances simulations significantly as compared to all-atom models.
The PACE model combines a united-atom (UA) force field, developed in our group, with the MARTINI CG model. Unlike most all-atom models, PACE is designed to be fully compatible with the MARTINI CG water and lipid models, enabling accurate predictions of solvation free energies for small solutes and their transfer free energies from water to membranes. Additionally, the UA protein components of PACE retain critical interaction features, such as hydrogen bonding (HB) and π–π stacking, and are calibrated to reproduce the statistical distribution of peptide conformers.
As a result, PACE has demonstrated the ability to preserve the native structures of several model proteins during simulations. Notably, it also facilitates ab initio folding of multiple small proteins. Furthermore, the robustness of the PACE model has been rigorously tested and validated through simulations of peptide self-assembly systems with a wide variety of structures, extensively characterized by experimental data. Finally, the PACE model has been successfully applied to studies of membrane protein functional mechanisms, showcasing its versatility and potential for advancing our understanding of complex biological systems.